COVID-19 Antibody Instant Test Kit
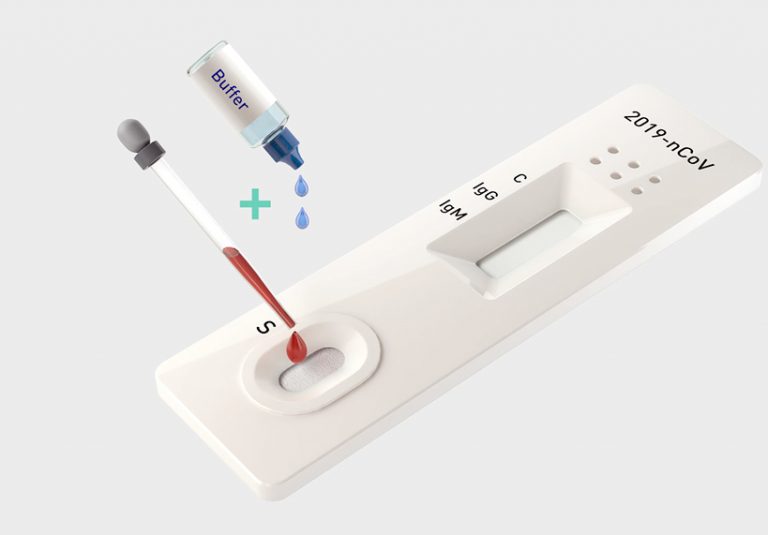
How it works
1. Results in 10 minutes
2. Small sample sizes
3. Accurate analysis
4. Sold in packs of 25
5. Made in USA inventory available
6. Shelf life of up to 24 months from manufacture date
7. CE marking (conforms with health standards for products sold within the European Economic Area)
TMED Supply is now offering COVID-19 Antibody Instant Test Kit.
To purchase or request more information about these COVID-19 Antibody Instant Test Kits, such as clinical trial info, the product insert, and CE documentation, please complete the form below.
Sold in packs of 25 tests, minimum of 1000 tests required. International orders above 10,000 units will be given priority due to extremely high demand. Thank you for your understanding.
COVID-19 Antibody Instant Test Kit
The test manufactured by Healgen Scientific LLC is conducted by using whole blood/serum/plasma and placing it in the center well of the cassette and then adding two drops of the buffer to the buffer well labeled “B.” Results should appear within 10 minutes and are invalid after 15 minutes. For more information, watch the video above.
Clinical Evaluation Results:
The clinical evaluation was carried out during February 2020 across 10 hospitals and reported on March 12, 2020. Further clinical evaluations are ongoing and any further information needed can be granted at request.
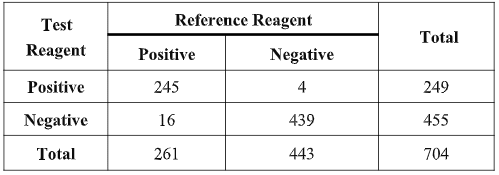
The results from the Healgen Scientific LLC tests show that the testing reagent and reference reagent have equivalent effectiveness in detecting COVID-19 when tested in the same clinical specimens.
Compared with the reference reagent, the positive agreement was 93.87% (95%CI:90.24%~96.46%), the negative agreement was 99.10% (95%CI:97.70%~99.75%) and total agreement was 97.19% (95%CI:95.65%~98.26%).
The kappa value of the consistency analysis was 0.94 (95%CI:95.65%~98.26%).
The results of the clinical evaluation show that the two reagents (methods) have a high degree of consistency and equivalent sensitivity and specificity in detecting COVID-19.
Product Facts:
1. Approved for sale in EU and Internationally
2. Made in USA inventory available
3. Following the incubation period, IgM may appear in blood within 3-5 days.
4. IgG will appear as soon as 1-2 weeks.
5. Sample: Test can work with whole blood, plasma and serum samples.
6. Storage: The kit can be stored at room temperature or refrigerated (2-30°C).
7. Shelf Life: Up to 24 months from manufacture date
More information, such as the product insert, clinical trial results, and CE documentation is available at request
COVID-19 IgG/IgM Rapid Test Cassette Instructions for Use

1. Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface.
3. (For Whole Blood Specimen): Hold the 5 μL mini plastic dropper vertically and transfer 1 drop of whole blood (about 10 μL) to the specimen well(S) of the test device, then add 2 drops (about 80 μL) of sample buffer to the buffer well (B) immediately. Avoid air bubbles.
(For Serum or Plasma Specimens): With a 5 μL mini plastic dropper provided, draw serum/plasma specimen to exceed the specimen line as showed in the following image and then transfer drawn serum/plasma specimen into the sample well (S). Then add 2 drops (about 80 μL) of sample buffer to the buffer well (B) immediately. Avoid air bubbles.
Wait for the colored line(s) to appear. The result should be read in 10 minutes. Positive results may be visible as soon as 2 minutes. Do not interpret the result after 15 minutes.
Interpretation of Results:
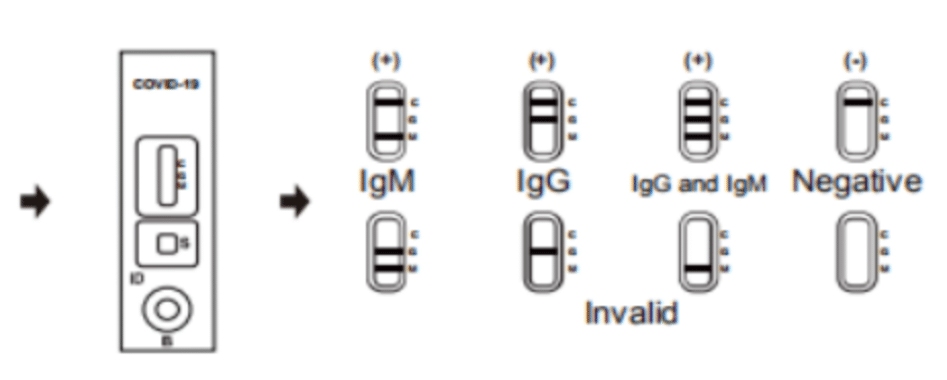
Negative: If only the C band is present, the absence of any burgundy color in the both T bands (IgG and IgM) indicates that no anti-COVID-19 antibodies are detected in the specimen. The result is negative.
IgM Positive: In addition to the presence of C band, if only IgM band is developed, the test indicates for the presence of IgM anti-COVID-19 in the specimen. The result is IgM anti-COVID-19 positive.
IgG Positive: In addition to the presence of C band, if only IgG band is developed, the test indicates for the presence of IgG anti-COVID-19 in the specimen. The result is IgG anti-COVID-19 positive.
IgG and IgM Positive: In addition to the presence of C band, both IgG and IgM bands are developed, the test indicates for the presence of both IgG and IgM anti-COVID-19 in the specimen. The result is IgG and IgM anti-COVID-19 positive.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
