Coronavirus Ag Rapid Test Cassette (Swab) (Antigen Test Kit)
How it works
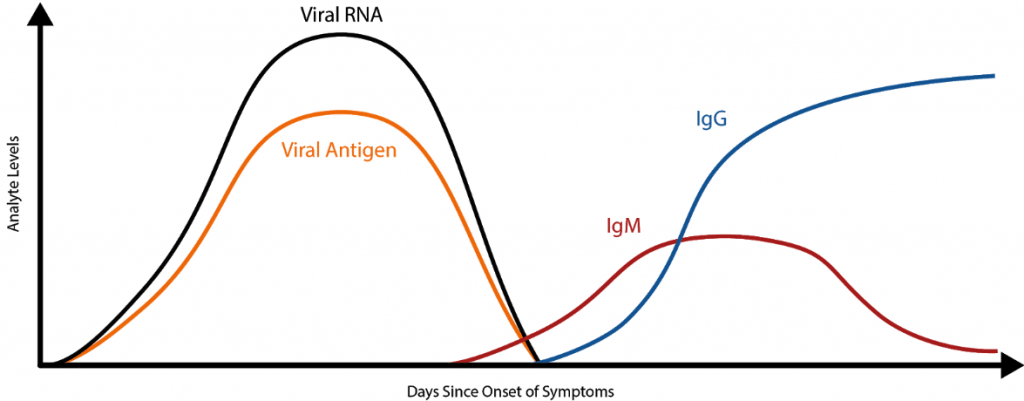
The Coronavirus Ag Rapid Test Cassette (Swab) can detect acute infection in a fraction of the time as a molecular (PCR) test and are far less expensive. This option is perfect for when you want to detect an infection early, before the body has had a chance to produce antibodies.
1. Results under 15 minutes
2. CE Marked, International Only (Not to be used or sold in the USA)
3. Accurate diagnostic tool for acute detection
4. Nasal or throat swab collection
5. Easy to administer and read results
6. For professional use only
7. For in vitro diagnostic use only
8. 24 month shelf life, do not use after expiration date
9. Store at room temperature or refrigerated (2-30°C)
10. Sold in packs of 20
TMED Supply is now offering Coronavirus Ag Rapid Test Cassette (Swab) test kits.
If you are interested in this product, please complete the form below and one of our representatives will reach out to discuss the details. Currently, this product is available for South Africa.
Clinical Sensitivity, Specificity and Accuracy
The Coronavirus Ag Rapid Test Cassette (Swab) has been evaluated with specimens obtained from patients. A commercialized molecular assay was used as the reference method. The results show that the Coronavirus Ag Rapid Test Cassette (Swab) has a high overall relative accuracy.
Coronavirus Ag Rapid Test Cassette (Swab) Product Details
Intended Use, Summary and Explanation
The Coronavirus Ag Rapid Test Cassette (Swab) is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal (NP) swab specimens directly or after the swabs have been added to viral transport media from individuals who are suspected of COVID-19 by their healthcare provider. It is intended to aid in the rapid diagnosis of SARS-CoV-2 infections. The Coronavirus Ag Rapid Test Cassette (Swab) does not differentiate between SARS-CoV and SARS-CoV-2.
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
This test is for detection of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.
Principle Of The Test
The Coronavirus Ag Rapid Test Cassette (Swab) is an immunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to detect nucleocapsid protein from SARS-CoV-2 in nasopharyngeal (NP) swab. The test strip is composed of the following parts: namely sample pad, reagent pad, reaction membrane, and absorbing pad.
The reagent pad contains the colloidal-gold conjugated with the monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2; the reaction membrane contains the secondary antibodies for nucleocapsid protein of SARS-CoV-2. The whole strip is fixed inside a plastic device.
When the sample is added into the sample well, conjugates dried in the reagent pad are dissolved and migrate along with the sample. If SARS-CoV-2 antigen presents in the sample, a complex formed between the anti-SARS-2 conjugate and the virus will be captured by the specific anti-SARS-2 monoclonal antibodies coated on the test line region (T).
Absence of the T line suggests a negative result. To serve as a procedural control, a red line will always appear in the control line region (C) indicating that proper volume of sample has been added and membrane wicking has occurred.
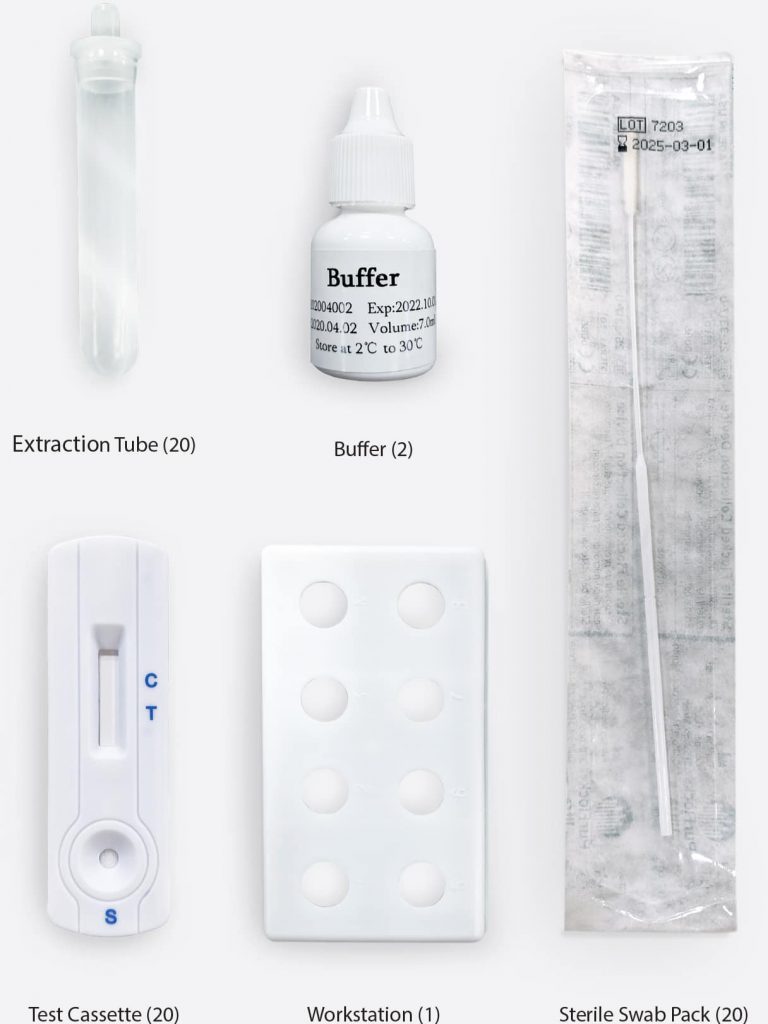
Coronavirus Ag Rapid Test Cassette (Swab) Test Pack Contents
Each box of Coronavirus Ag Rapid Test Cassette (Swab) test kits includes:
20 Test cassettes
20 Sterile swabs
20 Extraction tubes and dropper tips
1 Workstation
2 Buffers
1 Package insert
How To Use The Coronavirus Ag Rapid Test Cassette (Swab) Test Kit
Prior to use, please review and follow the manufacturer’s product insert included with this product.
Specimen Collection
Use the nasopharyngeal swab supplied in the kit.
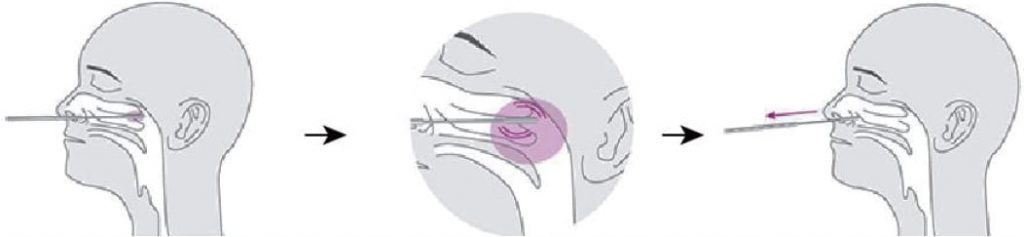
1. Carefully insert the swab into the nostril of the patient, reaching the surface of posterior nasopharynx. that presents the most secretion under visual inspection.
2. Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
3. Withdraw the swab from the nasal cavity.
Sample Preparation Procedure
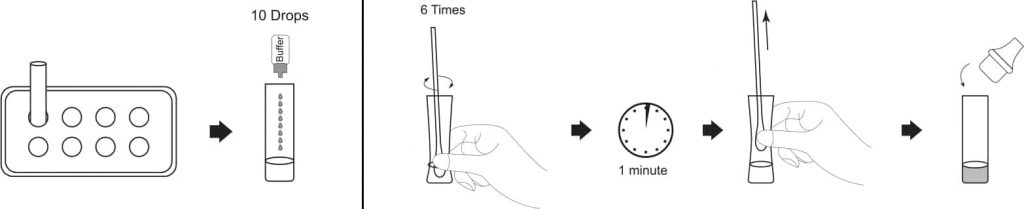
1. Insert the test extraction tube into the workstation in this product. Make sure that the tube is standing firm and reaches the bottom of the workstation.
2. Add 0.3 mL (about 10 drops) of the sample extraction buffer into the extraction tube.
3. Insert the swab into the extraction tube which contains 0.3 mL of the extraction buffer.
4. Roll the swab at least 6 times while pressing the head against the bottom and side of the extraction tube.
5. Leave the swab in the extraction tube for 1 minute.
6. Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab. The extracted solution will be used as test sample.
Specimen Transport and Storage
Specimens should be tested as soon as possible after collection. If transport of samples with viral transport medium (VTM) is required, minimal dilution of the sample is recommended, as dilution may result in decreased test sensitivity. Whenever possible, 1 milliliter or less is best to avoid excessive dilution of the patient sample. While holding the swab, remove the cap from the tube.
Insert the swab into the tube until the breakpoint is level with the tube opening. Bend the swab shaft at a 180 degrees angle to break it off at the breaking point. You may need to gently rotate the swab shaft to complete the breakage. Based on data generated with influenza virus, or nasopharyngeal swabs in VTM are stable for up to 72 hours at 2° to 8°C.
Note: When using viral transport medium (VTM), it is important to ensure that the VTM containing the sample is warmed to room temperature. Cold samples will not flow correctly and can lead to erroneous or invalid results. Several minutes will be required to bring a cold sample to room temperature.
Test Procedure
Allow the test device, test sample and buffer to equilibrate to room temperature (15-30°C) prior to testing.
1.Remove test device from the sealed pouch just prior to the testing and lay flat on work bench.
3. Insert a nozzle with filter into the sample extraction tube tightly.
4. Reverse the sample extraction tube, and add 4 drops (about 100 μL) of test sample by squeezing the extracted solution tube into the sample window.
5. Wait for the colored band(s) to appear. The result should be read in 15 minutes. Do not interpret the result after 20 minutes.
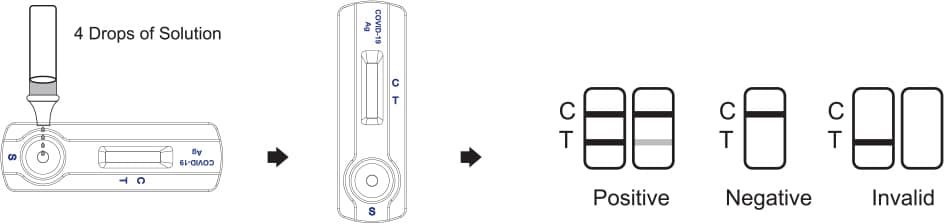
Interpretation Of Results
POSITIVE
The presence of two lines as control line (C) and test line (T) within the result window indicates a positive result.
NEGATIVE
The presence of only control line (C) within the result window indicates a negative result.
INVALID
If the control line (C) is not visible within the result window after performing the test, the result is considered invalid. Some causes of invalid results are because of not following the directions correctly or the test may have deteriorated beyond the expiration date. It is recommended that the specimen be re-tested using a new test.
NOTE:
1. The intensity of color in the test line region (T) may vary depending on the concentration of analyses present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive. Please note that this is a qualitative test only, and cannot determine the concentration of analytes in the specimen.
2. Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
More About COVID-19 Antigen Testing
Antigen tests are faster and less expensive than molecular tests (PCR), therefore, some experts consider them to be more feasible for coronavirus testing of large numbers of people. However, antigen tests may not detect all active infections, as they do not work the same way as a PCR test.
Instant coronavirus antigen tests are very specific for the virus, but not as sensitive as molecular PCR tests. Positive results from antigen tests are highly accurate, but there is a higher chance of false negatives when compared to PCR tests. Therefore, multiple antigen testing sessions at different intervals are recommended.
This newer method of COVID-19 detection identifies certain proteins that are representative of the virus. Using a nasal or throat swab to get an adequate fluid sample, antigen tests can provide results in minutes, and are useful in detecting acute infection of COVID19/SARS.